
COWIN Calcium Chloride Production Methods

| This section covers some general principles for production of calcium chloride and gives an overview of the production processes utilised by COWIN Industry Limited . |
Basic Production Processes
Calcium chloride is produced mainly by two different processes—the limestone-hydrochloric acid and the natural brine process. The selection of process is usually determined by the supply of raw materials and transportation and energy costs.
To read a brief overview of each process, click on one of the following links: |
a
| Limestone-Hydrochloric Acid Process |
|
![]() |
iLimestone can be treated with hydrochloric acid to form calcium chloride and carbon dioxide:
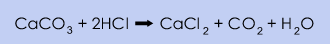
If concentrated (36%) hydrochloric acid is utilised, the concentration of CaCl2 in the produced solution will be approximately 40% and further evaporation is only needed when flakes are produced. The purification of the product is mainly accomplished by adding Ca(OH)2 as described in the natural brine process.
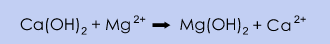
The high purity of the produced carbon dioxide (CO2) makes it suitable for applications within the food and pharmaceutical industries e.g., in the production of carbonated soft drinks.
The limestone process is utilised for calcium chloride production in our Production Base Binhai District (view process), Jinnan (view process) and Linyi (view process) facilities.
The limestone comes from Shandong Province and the hydrochloric acid is produced at neighbouring chemical plants.
Extremely pure calcium chloride products can be obtained using this process if the purity of the hydrochloric acid and limestone is sufficient. Hence, the process is well suited for producing food grade products. It is also an environmentally friendly method to utilise by-product hydrochloric acid.
| Natural Brine Process |
|
![]() |
In the U.S., production is utilised by the concentration and purification of naturally occurring brines from salt lakes and salt deposits. Magnesium is removed by adding milk of lime, Ca(OH)2, which causes magnesium to precipitate in the form of magnesium hydroxide, Mg(OH)2. Sodium chloride, NaCl, is removed by precipitation; sodium chloride precipitates from the CaCl2 solution when the concentration of calcium chloride is increased during the evaporation of water.
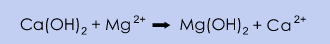
The advantage of this source of calcium chloride is the low raw material cost and low environmental impact. However, the purity of the product is normally lower than the competing processes.
| The Solvay Process |
|
![]() |
In China , there are many plants production is utilised by the concentration and purification of liquid from Solvay Process of soda ash plant .
|